Originally from Cuba, Lazaro Peraza (also known as Laz) is a senior pursuing dual degrees in Neuroscience and Human Biology. He is in the process of applying to medical school and a few master’s programs. He is also one of the co-presidents of USC’s very own Break On 2: Latin Fusion salsa team. After graduation, Laz plans to travel and volunteer abroad in Cuzco, Peru with USC MEDLIFE and obtain his EMT certification. Laz also wants to continue his current research projects at USC’s Imaging Genetics Center, where he conducts research on Alzheimer’s disease and other forms of dementia. He enjoys salsa dancing and surfing, and also plays soccer when he isn’t drowning in school work. He is an avid FC Barcelona fan and absolutely despises Real Madrid C.F. Once accepted into medical school, he plans on pursuing an M.D/J.D dual professional degree.
____________________________________________________________________________________________________________________________________________________
Vitamin C as a Future Adjunctive to Cancer Therapy
Lazaro Peraza
[Introduction]
In the 1970’s, world-renowned scientist Linus Pauling proposed a new approach to terminal cancer treatment, suggesting high dosages of vitamin C were an inexpensive, yet effective, adjunctive to cancer therapy. He believed vitamin C would reduce the effects of therapy-induced side effects and increase patient longevity. However, as subsequent studies failed to replicate his results, Pauling’s theory was quickly disregarded and further research in the field halted. Although it remained an under-investigated subject, new research over the next thirty years concluded that the studies invalidating Pauling’s theory were not accurate replications of the initial experiment – they examined the effect on cancer patients of orally administered vitamin C without also including the effect of intravenous administration of the same substance. In light of this finding, research was resumed, and recent studies in pharmacokinetics have now conclusively verified that the effective concentration level of vitamin C in the blood can only be attained through an intravenous injection.
While it can no longer be argued whether or not vitamin C plays a role in cancer treatment, the ongoing debate among the medical research community questions whether that role is positive or negative due to convincing research supporting both sides of the argument. However, an increasing number of experiments are documenting results that verify the beneficial effects of vitamin C, including tumor metastasis control and the reduction of therapy-induced side effects. Divergent findings currently prevent vitamin C from being pursued on an administrative scale, but the potential of vitamin C to serve as a positive addition to cancer treatment demands further research to determine its future as an alleviator to the existing aggressive and toxic therapies currently in practice.
[Mayo Clinic Trial & Intravenous Vitamin C]
While the revitalization of vitamin C research can arguably be accredited to the findings of Sebastian J. Padayatty in 2006, its multi-decade hiatus can largely be attributed to the failed trials of the Mayo Clinic during their attempt to replicate Pauling’s findings a few years after the publication of his paper. The highly esteemed Clinic’s negative results concluding that vitamin C played no beneficial role in cancer treatment, and could even prove detrimental in some cases (Creagan, E. et al., 1979; Padayatty, S. et al., 2006), soon drew additional critiques against Pauling’s study. His experiment was reproved for being terribly organized and for not accounting for the appropriate controls, standardizations, stage of cancer, or even patient progress. It appeared as if the entire study was setup in order to draw attention to his theory, and his experimental protocols as a whole were deemed subpar (Respectful Insolence, 2009). However, in reality, the much-lauded Mayo Clinic studies were no better. In fact, they did not mimic Pauling’s trials as closely as they claimed.
While Pauling’s experiment documented results for high and frequent doses of vitamin C administered both orally and intravenously, the Mayo trials failed due to only administering doses of vitamin C orally, completely disregarding dose administration via IV injection (Padayatty, S. et al., 2006). Recent findings in pharmacokinetics, the study of the fate of externally administered substances, have reported that oral doses of vitamin C, such as those used by the Mayo Clinic, are incapable of increasing plasma levels in the human body to a high enough level to have any effect, regardless of their size or frequency (Hornig, 1975; Kuether, et al., 1988). This incapability stems from the water-solubile properties of Vitamin C which limit the maximum amount of bodily absorption possible when ingested orally. Vitamin C plasma levels in normal, healthy adults do not exceed a concentration of 100 μmol/L. Findings on oral doses only show minimal increases in those concentrations ranging from 70 μmol/L to 220 μmol/L – fluctuations too small to be either detrimental or beneficial (Padayatty, S. et al., 2006). In contrast, IV administration has been shown to have drastically higher absorption rates, increasing plasma concentrations up to 14,000 μmol/L due to the antioxidant being incorporated directly into the bloodstream (Levine, M. et al., 1996; Padayatty, S. et al., 2006). Although these statistics alone do not single-handedly vindicate Pauling’s findings, they do provide compelling grounds for further investigation given the fact that concentrations of plasma ranging from 1,000 to 5,000 μmol/L have proven to be both selective and highly destructive to cancer cells – levels that can only be reached via intravenous dosages of vitamin C (Padayatty, S. et al., 2006).
[Case Studies]
Based on the previous evidence, Sebastian J. Padayatty and his associates began further research on the subject in 2006. He and his team began analyzing the cases of terminal cancer patients who chose intravenous vitamin C as their primary cancer treatment. In all three of the cases they studied, the patients exhibited dramatic responses to high-dose, intravenous administration of vitamin C. Each patient experienced complete remission of the cancer from their original diagnosis, with two of the three patients going on to live full, cancer-free lives post ascorbic acid therapy[FK1] . Oddly enough, the three patients had a wide variety of cancer types that proved responsive to vitamin C therapy. Their diagnoses are as follows: Patient 1, a fifty-one year old woman, was diagnosed with renal cell carcinoma and lung metastasis; Patient 2, a forty-nine year old male, was diagnosed with a primary bladder tumor along with multiple satellite tumors surrounding it; Patient 3, a sixty-six year old female, was diagnosed with large B-cell lymphoma with evidence of bone invasion. While the studies indicated varying supplemental products such as beta-carotenes and bioflavonoids in order to complement treatments, each patient received only intravenous vitamin C as their primary cancer therapy and did not receive any chemotherapy pre- or post-diagnosis and ascorbic acid administration. Patients 2 and 3 underwent follow-up examinations ten years after to their initial diagnoses and, as stated above, remained in good health with no signs of metastasis. Patient 1 also experienced complete remission of her renal cell cancer and discontinued additional treatments for the next four years as a result. She ended up passing away in 2002 after being diagnosed with small-cell lung cancer, however, additional health complications surrounding the second diagnosis caused these secondary results to be discarded (Padayatty, S. et al., 2006).
The favorable responses observed in patients 1 and 2, and the partial positive response observed in patient 3 do not by any means prove conclusively that ascorbic acid was exclusively responsible for the patients’ optimistic outcomes; however, they did spearhead practitioners to begin using ascorbic acid therapy in clinical trials on a much larger scale. In fact, from 2006 to 2008, over ten thousand patients have received high doses of IVC without experiencing ill complications (Padayatty, et al., 2010); instead, an overwhelming majority of patients treated worldwide during that timeframe have reported that IVC, when combined with their standard therapy, has drastically reduced side-effects including nausea, vomiting, and loss of appetite, thus enhancing their quality of life (Yeom, et al., 2007). Observing results of this nature on a large scale has spurred scientists to seek an understanding of the mechanism by which vitamin C has proved to be beneficial in cancer eradication. In order to do this, researchers have been forced to take a step back and examine the entire process of cancer proliferation and metastasis, beginning with cancer cell division, on a molecular level.
[Cancer Cell Proliferation]
When DNA in a normal cell is damaged – which could result from any range of activities from replication errors to cigarette smoking – it is possible that programmed death cell, or apoptosis, will become compromised due to mutation. These mutated cells, now referred to as cancer cells, then activate cellular mechanisms which cause rapid cell division at rates higher than normal cells and spread throughout the body in a process known as metastasis. The clustering of anomalous cancer cells results in the formation of tumors that can grow in most parts of the body. Tumors induce new blood vessel growth via angiogenesis through secretion of necessary growth factors, and then in turn receive their nutrients from those newly formed blood vessels. Together, these mechanisms allow rapid proliferation of cancer cells and allow tumors to reproduce and increase in size at an alarming rate, unchecked by regulatory cellular functions. These tumors may require varied treatments depending on their location, size, and rate of growth. The three main forms of treatment for these malignant tumors are surgery, radiation, and chemotherapy.
[Treating Cancer]
Localized malignant tumors, or cancer cells that are restricted to one area, are removed by invasive surgery. A surgical procedure involves safely removing the tumor as well as any tissue surrounding it thought to contain damaged cells, in order to prevent reoccurrence (Cancer Society, 2013). While radiation therapy can be used as an independent treatment for localized cancer, it is frequently used pre- and post-surgical procedures to either shrink a tumor into operable size before surgery, or to damage or destroy any remaining cancer cells afterward. In cases where the cancer has spread to various parts of the body as opposed to being localized, however, chemotherapy is used (Cancer Society, 2013).
Chemotherapy, or chemo for short, has rapidly become the most effective and versatile method of cancer treatment as it produces a variety of positive results, such as reducing the size of a tumor before surgical removal, assisting in remission, and slowing cancer cell growth (Cancer Society, 2013). Chemo works through introducing drugs, which travel through the bloodstream to reach multiple areas of the body – a unique trait that sets it apart from surgery and radiation. Nonetheless, despite its undeniable effectiveness, chemo has many aggressive side effects. Many times after a cycle, the patient may experience therapy-induced vomiting, nausea, hair loss, and diarrhea (Cancer Society, 2013). These symptoms arise from the primary flaw of chemo: the administered drugs not only kill cancerous cells, they also kill healthy cells as well[FK2] . Due to a lack of selectivity, these drugs do not have a way to specifically target the abnormal cells in the body. In fact, most of these drugs generate high levels of oxidative stress by way of reactive oxygen species (ROS), which are known to be cytotoxic, or directly harmful to normal cell function (Cancer Society, 2013).
Reactive Oxygen Species, or free radicals, are a natural metabolic by-product in the human body, and many different catalases, peroxidases, and superoxide demutases, as well as a range of antioxidants, including vitamin C, monitor their normal levels. Chemotherapy actively takes advantage of the toxicity of free radicals by flooding the body with excessive levels, ultimately damaging both healthy and abnormal cells. However, as mentioned above, antioxidants such as vitamin C typically serve to monitor and stabilize ROS levels in a healthy human being, acting as scavengers for these free radicals, detecting and killing them. Therefore, scientists including David Golde, M.D., a physician at Memorial Sloan-Kettering Cancer Center in New York, argue that based on its natural function, vitamin C would interfere with chemotherapeutic treatments by reducing their efficacy, thus proving to be detrimental (Gottlieb, N. 1999).
[Mechanism Proposals]
Studies supporting this view have been conducted, albeit in vitro, and have concluded that vitamin C was actually helping tumor cells. For example, in 2008, scientist Shannon Kirkey led a study on mice that were given regular chemotherapies. Her control group showed significant tumor size reduction, while her experimental group, which was administered vitamin C as an adjunctive to chemo, showed tumors that were four to five time larger than their counter parts when compared four weeks later. Kirkey and like-minded scientists argue that her results demonstrate that vitamin C should not be used in conjunction with chemotherapy (Heaney, et al., 2008; Kirkey, S. et al., 2008). However, after taking a closer examination at the study and those similar, alternate research groups have discovered that the overwhelming majority of all studies showing detrimental effects of IVC have used dehydroascorbic acid (the oxidized version of vitamin C) as opposed to ascorbic acid (the reduced form of vitamin C) (Espey, et al., 2009). Those studies that were conducted using ascorbic acid only reported wide scale positive results as detailed above.
The widely divergent findings reported in studies using oxidized vitamin C versus those using reduced vitamin C led scientist to further question the mechanism by which ascorbic acid was able to facilitate positive results. Kedar N. Prasad, Ph.D., a radiology professor at the University of Colorado Health Sciences Center, is only one of many who are currently researching this subject (Gottlieb, N. 1999). He believes ascorbic acid to have selective properties able kill and reduce cancerous cells at tumor sites. Prasad is currently testing this hypothesis, but he believes the demonstrated selectivity is due to homeostatic controls. He further theorizes that while normal cells can functionally regulate the uptake of ascorbic acid, tumor cells cannot. Without regulation, tumor cells accumulate massive amounts of antioxidants and create a genetic cascade resulting in programmed cell death of the mutated cells (Gottlieb, N. 1999). Prasad now favors incorporation of antioxidants in cancer treatments, as he believes they serve as beneficial supplements.
[Ascorbic Acid Selectivity]
The selectivity mechanism supported by Prasad is known as the Fenton Reaction, which involves metal ions reacting with a reducing agent, such as vitamin C, to create cell damage. Many scientists, including the aforementioned David Golde, claim this reaction can only happen in vitro (and not in vivo) due to a lack of sufficient free metal ions in the body (Gottlieb, N. 1999). Prasad, however, states that there are ample free metal ions located in both blood plasma and many organs for the reaction to take place in vivo. In bold fashion Prasad states, “It is an experimental fact, it is a clinical fact, that antioxidants at high doses are selective for inhibiting the growth of cancer cells” (Gottlieb, N. 1999). The lack of clinical data in vivo, nonetheless, is a primary factor to why many scientists refuse to believe vitamin C is a beneficial adjunctive. While Prasadian theories are not advanced enough be conducted on human subjects in order to refute naysayers, the Riordan Clinic and Research Institute has spearheaded in vitro, ex vivo, and in vivo trials in a variety of different animal species with positive results.
One such study conducted by the Riordan Clinic Research Institute, as funded by the National Institute of Health, examined mice as they were implanted with sarcomas, a species of malignant tumor. Results detailed control mice surviving an average of 35.7 days post implantation, as opposed to the vitamin C treated mice who survived on average 50.7 days (Yeom, C. et al., 2009). The Clinic also provided Figure 9 below to support findings.
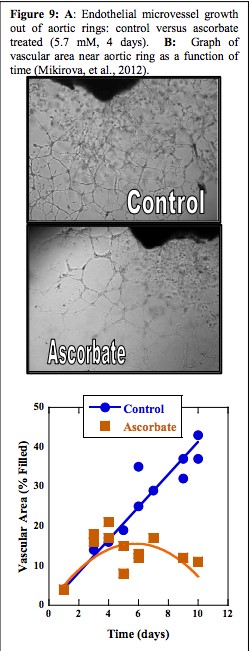 Figure 9A (Mikirova, et al., 2008; Mikirova, et al., 2012) shows a comparison between the blood vessel growth in both control and ascorbate treated endothelial cells taken from aortic rings – abnormal formations of the blood vessels surrounding the aorta. The noticeable difference in the number of vessels depicted suggests that vitamin C has an inhibitory effect on the formation of new vascular tubules from pre-existing tubules. Tumor angiogenesis is crucial in metastasis as it is a key reason for the capability of tumors to spread and grow with such rapidity: they receive mass mounts of essential nutrients from these newly formed vessels, which speeds growth.
Figure 9A (Mikirova, et al., 2008; Mikirova, et al., 2012) shows a comparison between the blood vessel growth in both control and ascorbate treated endothelial cells taken from aortic rings – abnormal formations of the blood vessels surrounding the aorta. The noticeable difference in the number of vessels depicted suggests that vitamin C has an inhibitory effect on the formation of new vascular tubules from pre-existing tubules. Tumor angiogenesis is crucial in metastasis as it is a key reason for the capability of tumors to spread and grow with such rapidity: they receive mass mounts of essential nutrients from these newly formed vessels, which speeds growth.
Similarly, figure 9B is a graph comparing the vascular area near the aortic rings as a function of time between the control and ascorbate treated cells. As shown, the ascorbate treated cells occupied much less space than the control cells, further supporting the recent theory that without angiogenesis, tumors cannot grow (Mikirova, et al., 2008; Mikirova, et al., 2012). Accumulation of similar data would provide conclusive evidence that tumor angiogenesis can be inhibited by vitamin C administration. Since these discoveries, the Riordan Clinic has continued research on vitamin C and incorporated it the services provided by their clinic. Although stringent requirements must be met in order to receive IV vitamin C in conjunction with chemotherapy, cancer patients from all over the world seek consultation and treatment at the Riordan Clinic in Kansas.
In conclusion, when Linus Pauling initially introduced vitamin C as an adjunctive to cancer therapy, he had no idea that it would spur not only a long lasting debate among the medical community, but that it would also inspire a new generation of research. Although there was not enough evidence to confirm or conclusively justify his claim at the time of his hypothesis, his theory is finally being developed into a much needed alleviator of the aggressive effects of chemotherapies over forty years later. Clinical cancer patients have now been provided with a method to better fight the deadly disease, their positive results providing hope for a gentler treatment and possible long-term cure. While the evidence supporting IVC administration, specificity mechanisms, and prevention of angiogenesis alone will not cure terminal cancer, they do represent monumental, milestone achievements in science and present researchers with a new building block upon which to base further study and the future of cancer treatment.
Works Cited
Berlin, S. et al., 2009. Antiproliferative effect of ascorbic acid is associated with inhibition of genes necessary for cell cycle progression. PLoS ONE, Volume 4, pp. E44-0.
Cameron, E. & Pauling, L., 1976. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. PNAS USA, Volume 73, pp. 3685-9.
Cameron, E., Pauling, L. & Leibovitz, B., 1979. Ascorbic acid and cancer, a review. Cancer Res, Volume 39, pp. 663-81.
Cancer Society. 2013. Making treatment decisions. http://www.cancer.org/treatment/understandingyourdiagnosis/afterdiagnosis/after-diagnosis-making-treatment-decisions
Cancer Society. 2013. What is cancer? http://www.cancer.org/cancer/cancerbasics/what-is-cancer
Chen, Q. et al., 2008. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. PNAS USA, Volume 105, pp. 11105-9.
Creagan, E. et al., 1979. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer: A controlled trial. NEJM, Volume 301, pp. 687-690.
Espey, M., Chen, Q. & Levine, M., 2009. Comment re: vitamin C antagonizes the cytotoxic effects of chemotherapy. Cancer Research , Volume 69, p. 8830.
Gottlieb, N. 1999. Cancer treatment and vitamin C: The debate lingers. Journal of the National Cancer Institute, 91(24), 2073-5.
Heaney, M. et al., 2008. Vitamin C antagonizes the cytotoxic effects of antineoplastic drugs. Cancer Res., Volume 68, pp. 8031-8.
Hornig, D., 1975. Distribution of ascorbic acid metabolites and analogues in man and animals. Ann NY Acad Sci, Volume 258, pp. 103-18.
Kirkey, S. et al., 2008. Vitamin C impedes chemo: Study; in petri dishes, vitamin helped keep cancer cells alive. The Ottawa Citizen.
Kuether, C., Telford, I. & Roe, J., 1988. The relation of the blood level of ascorbic acid to tissue concentrations of this vitamin and the histology of the incisor teeth in the guinea pig. J Nutrition, Volume 28, pp. 347-58.
Levine, M. et al., 1996. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. PNAS USA, Volume 93, pp. 3704-9.
Mikirova, N., Ichim, T. & Riordan, N., 2008. Anti-angiogenic effect of high doses of ascorbic acid. J Transl Med, Volume 6, p. 50.
O’Connor, M.K., Malone, J.F., Moriarity, M., Mulgrew, S. (1977) A radioprotective effect of vitamin C observed in Chinese hamster ovary cells. Br. J. Radiol. pp. 587–591
Padayatty, S. et al., 2006. Intravenous vitamin C as a cancer therapy: three cases. CMAJ, Volume 174, pp. 937-42.
Padayatty, S. et al., 2010. Vitamin C: intravenous use by complementary and alternative medical practitioners and adverse effects. PLoS ONE, Volume 5, p. 11414.
Padayatty, S. et al., 2004. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann. Intern. Med., Volume 140, pp. 533-37.
Science Blogs. 2009. Respectful Insolence. Retrieved March 27, 2014, from http://scienceblogs.com/insolence/2009/02/18/vitamin-c-and-cancer-has-linus-pauling-b/
Yeom, C., Jung, G. & Song, K., 2007. Changes of terminal cancer patients health related quality of life after high dose vitamin C administration. Korean Med Sci, Volume 22, pp. 7-11.
Yeom, C. et al., 2009. High-dose concentration administration of ascorbic acid inhibits tumor growth in BALB/C mice implanted with sarcoma 180 cancer cells via the restriction of angiogenesis. J Transl Med, Volume 7, p. 70.




Leave a Reply